

With the exception of groups 312 (the transition metals ), the units digit of the group number identifies how many valence electrons are associated with a neutral atom of an element listed under.
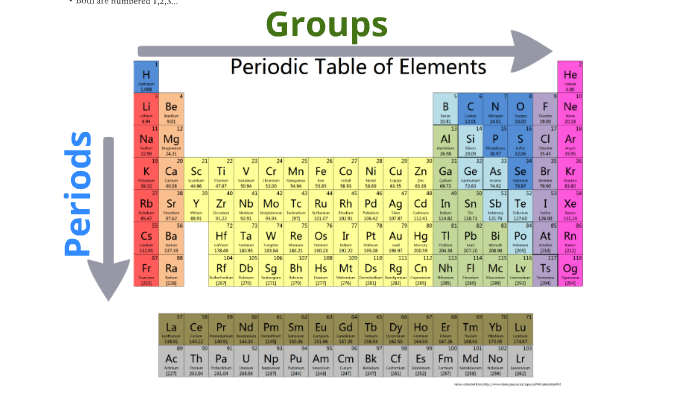
Group 1: The Alkali Metals lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr) are soft, shiny, and highly reactive metals. The number of valence electrons of an element can be determined by the periodic table group (vertical column) in which the element is categorized. Chlorine atoms bridge Al centers in aluminum chloride dimer at left while the structure of pentaborane nonahydride shown at right includes four B-H-B bridge bonds arranged around the open face of a cluster of five B atoms held together by cluster bonds. For example, the elements of Group 1 are known as the alkali metals, Group 2 are the alkaline earth metals, Group 17 are the halogens, and Group 18 are the noble gases.


 0 kommentar(er)
0 kommentar(er)
